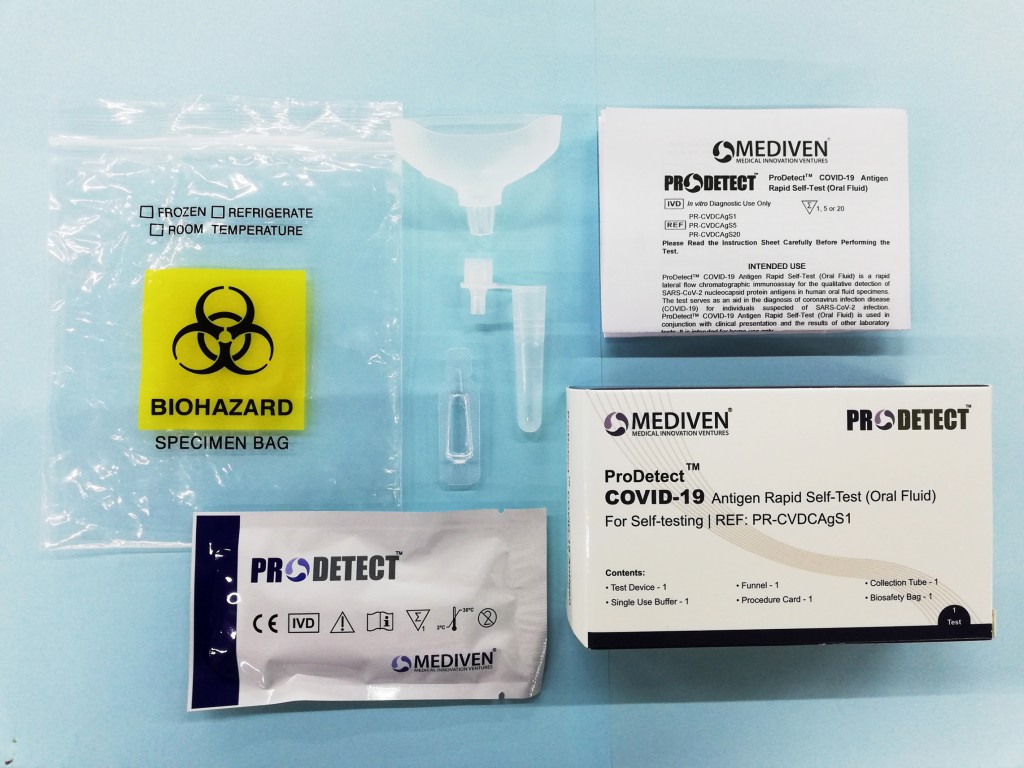
- Easy to use self-test oral fluid kit with high reliability and accuracy
- Able to detect Delta and other variants of concern
- Made in Malaysia
Following on from its recent Vaccin8 Programme launch, OMESTI Group’s Healthcare unit Bemed Tempua Sdn Bhd has signed with Mediven® [Medical Innovation Ventures Sdn Bhd] of Malaysia to supply the ProDetectTM COVID-19 Antigen Rapid Self-Test (Oral Fluid) Kits. The kits will be offered for sale via Bemed Tempua to OMESTI Healthcare’s corporate and retail customers across the country.
The Malaysian-made ProDetectTM COVID-19 Antigen Rapid Self-Test Kits received conditional approval from the Medical Device Authority (MDA) recently and already are in high demand, as companies and businesses across Malaysia rapidly ramp up their testing protocols in line with the new SOPs announced by the Government. The easy-to-use self-test kit is able to accurately screen for COVID-19 within 15 minutes.
Gerard Monteiro, of OMESTI Healthcare, says,
“We are proud to team up with Mediven®, to offer the ProDetectTM Test Kits to our customers. The high accuracy and reliability of these kits will be of major benefit to companies as they adopt frequent testing regimes to ensure the health and safety of their employees and workers. It will enable these businesses to resume normal operations more quickly and with greater confidence. The fact that the kits are made in Malaysia is a bonus!”
Each kit comes with a unique QR code which enables seamless tracking from manufacturer to end user, in line with MDA guidelines. Dr Lim Li Sze, Operations Director of Mediven®, says, “We are delighted to sign up with Bemed Tempua and OMESTI Healthcare as they continue with their drive to help businesses combat COVID-19 and protect their workforce.” The unique QR code included with each ProDetectTM kit enables customers to verify the authenticity of the kits they have purchased. “The QR code also ensures proper recording of patient information and prevents double reporting of the same patient result. This will help businesses and factories test and monitor all employees to be able to operate in a COVID-19-free environment,” Dr Lim adds.
The individually packed ProDetectTM Self-Test Kit has high accuracy of 97.0% with relative sensitivity of 90.1% and relative specificity of 99.3%. In an evaluation study carried out by TIDREC (Tropical Infectious Diseases Research & Education Centre) at Universiti Malaya, the ProDetectTM kits also scored 100% Positive Predictive Value (PPV) and 100% Negative Predictive Value (NPV), indicating extremely low probability of false positive or false negative results.
“Another very important characteristic of the ProDetectTM Test Kit is that its performance is not affected by the various spike protein and nucleocapsid protein mutations of the COVID-19 virus, in particular the highly contagious Delta variant. This means that possible infections with this and other variants are detected early on and safely contained," adds Dr Lim.
The ProDetectTM Test Kits are the latest addition to OMESTI Healthcare’s Vaccin8 and Screening Services portfolio. “Our Vaccin8 Programme has received a very positive response from the market and we are now helping companies to protect employees across the country with our screening services. Our medical teams are mobilised to carry out vaccinations and testing services on site at company premises, even in remote locations. This helps provide safety, convenience and flexibility while minimising disruption to business operations,” adds Mr Monteiro.
Ends
About Bemed Tempua / OMESTI Healthcare
Bemed Tempua Sdn Bhd, founded in 2006, is a registered healthcare provider, licensed by the Ministry of Health Malaysia. The company is engaged in the business of sales and distribution of pharmaceutical products in Malaysia. In July 2021, Bemed Tempua became a member of the OMESTI Group, a Malaysian technology solutions provider which recently diversified its portfolio into the healthcare sector with the launch of its Vaccin8 Programme and other related services. https://vaccin8.omesti.com www.omesti.com
About Mediven®
Medical Innovation Ventures Sdn. Bhd. (Mediven®) is a medical diagnostic company which develops, manufactures and markets advanced high quality clinical diagnostic molecular and rapid tests. With a vision to be a leading provider of personalised health through innovative diagnostics, Mediven® provides solutions for screening of a variety of infectious diseases including dengue, chikungunya, zika, malaria, influenza, tuberculosis and many more. Mediven®’s innovative efforts are supported by Malaysian Bioeconomy Development Corporation (Bioeconomy Corporation) and are aligned with the agency’s mission to nurture and champion biotechnology and bio-based companies in Malaysia that contribute to the people’s wellbeing.
The Mediven® product range includes the GenoAmp® Real-Time RT PCR SARS-CoV-2 Test Kit which has been approved for sale in the European Union (EU) since May 2020 and carries the CE mark. In an international third-party evaluation study (External Quality Assessment (EQA) / Proficiency Testing (PT)), conducted in October 2020 from the UK, the GenoAmp® Real-Time RT PCR achieved 100% concordance, alongside only a handful of 50 other brands from some 475 participating manufacturers from nearly 20 countries. www.mediven.com.my